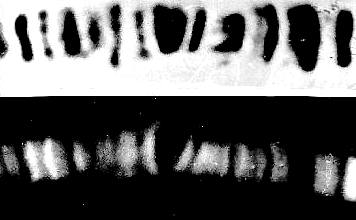
Giant chromosomes (polytene chromosomes) like this one from Drosophila melanogaster are characterized by a banding pattern. The bandings containing heterochromatin or histone-DNA-complexes, respectively, can easily be stained with conventional chromosome dyes (orcein in this case) and are thus simple to identify (upper picture). The in-between interbandings contain the active genes even though they consist of not very much DNA. The indirect immunofluorescence technique enables the detection of DNA-dependent RNA-polymerase required for transcription (lower picture; M. JAMRICH, A.L.GREENLEAF, E.K.F.BAUTZ, Heidelberg, 1977).
Specialized staining techniques for the staining of certain sections
of a chromosome (bandings) have been develop during the 1960th
and 70th. Depending on the pre-treatment of the chromosome and
the used dye or fluorochromes, it is distinguished between Q-, C-, G-,
and R-bandings. Most common are:
|
Q-bandings. Q-bandings result after treatment of the chromosomes with the fluorochrome quinacrin (= atebrin). They can be recognized by a yellow fluorescence of differing intensity. Most part of the stained DNA is heterochromatin. Quinacrin (atebrin) binds both regions rich in AT and in GC, but only the AT-quinacrin-complex fluoresces. Since regions rich in AT are more common in heterochromatin than in euchromatin, these regions are labelled preferentially. The different intensities of the single bands mirror the different contents of AT. Other fluorochromes like DAPI or Hoechst 33258 lead also to characteristic, reproducible patterns. Each of them produces its specific pattern. In other words: the properties of the bonds and the specificity of the fluorochromes are not exclusively based on their affinity to regions rich in AT. Rather, the distribution of AT and the association of AT with other molecules like histones, for example, has an impact on the binding properties of the fluorochromes. |
|
|
C-bandings. The name is derived from centromeric or constitutive heterochromatin. The preparations undergo alkaline denaturation prior to staining leading to an almost complete depurination of the DNA. After washing the probe the remaining DNA is renatured again and stained with Giemsa solution consisting of methylene azure, methylene violet, methylene blue, and eosin. Heterochromatin binds a lot of the dye, while the rest of the chromosomes absorb only little of it. The C-bonding proved to be especially well-suited for the characterization of plant chromosomes. |
|
|
G-bandings. G-bands are the result of a staining technique that is well-suited for animal cells but useless with plants. It resembles the C-banding technique without the pre-treatment. Plant chromosomes treated with this technique are uniformly stained. |
|
|
R-banding, reverse banding are the results of a technique that stains regions rich in GC that are typical for euchromatin. |
|
|
Hy-bandings. This method was developed especially for plant cells. They are treated with hot hydrochloric acid (HCl) and stained with acetic acid carmine. The pattern of Hy-bands is different from that of C-bands. It seems that the binding of protein to DNA and its more or less complete extraction has an impact on the binding ability of the acetic acid carmine. |
Variation, choice of further dyes and fluorochromes leads to an enhanced resolution of the banding techniques. Many non-modified attempts are well-suited for animal chromosomes but lead to considerable difficulties with plant chromosomes thus reducing the range of their use. The reasons are often not understood. The banding pattern of plant chromosomes does never reach the same degree of resolution common with animal chromosomes.
he consistent banding patterns of the constitutive heterochromatin and the remaining chromatin is strikingly constant in many species with an intraspecific variable karyotype. Intraspecific differences in the banding pattern were reported in Trillium-populations (I. FUKUDA and V. GRANT, 1980) and in polymorph Scilla-species (J. GREILHUBER and F. SPETA, Botanical Institute of the University of Vienna, 1977). In Scilla, the differences are minimal and are far outweighed by the similarities. D. SCHWEIZER and F. EHRENDORFER (also from the Botanical Institute of the University of Vienna) were able to distinguish the karyotypes of six different genera of Compositae easily, even though they are very similar in terms of structure and are very hard to distinguish with conventional staining. The analysis of the genus Scilla lead to an understanding of the changes of the chromosomal structure during adaptive radiation.
Often – but not always – the increase of DNA is caused by an increase of heterochromatin, i.e. on an increase of non-coding sections. In other cases, a decrease of the total amount of DNA was detected even though the amount of C-bandings increased. The evolution of new species is often accompanied by a restructuring of the heterochromatin.
Maize chromosomes display a number of ‘swellings’ or knobs that can be located in 23 different positions. Their number varies between 0 and 18 and is dependent on the variety. The chromosomes of varieties cultivated in the north of North America have no knobs, while varieties cultivated in southern North-America are especially rich in these knobs. The ‘knobs’ contain heterochromatin and can therefore be identified as C-bandings. The north-south divide illustrates that the amount of non-coding DNA, too, is subject to a strong selection pressure. Due to the shorter periods of vegetation in the North, varieties with less DNA have an advantage. The differences in the amount of DNA are as high as 37%. These observations indicated that even within a species, the genetic material is subject to severe restructuring (H. J. PRICE, Texas A&M University, College Station, 1987).
Chromosomal banding techniques have become valuable, though not always easy aids. Only few teams understand their effective use. No far-reaching and generalizing conclusions regarding the evolution of banding patterns and their importance for the evolution of plants have thus far been drawn. The majority of experiments were performed with monocots (Liliaceae and Gramineae). Only few dicots have large chromosomes displaying details after staining.
|
|