Medicinal Plant Use
in Africa
The
role of traditional medical practitioners
 |
| Photo
1. Trainee diviner
(twasa)
with a small quantity of
Boophane disticha
(Amarylldaceae) bulbs for
local use. |
|
In contrast with western
medicine, which is technically
and analytically based,
traditional African medicine
takes a holistic approach: good
health, disease, success or
misfortune are not seen as chance
occurrences but are believed to
arise from the actions of
individuals and ancestral spirits
according to the balance or
imbalance between the individual
and the social environment
(Anyinam, 1987; Hedberg et al.,
1982; Ngubane, 1987; Staugard,
1985; WHO, 1977). Traditionally,
rural African communities have
relied upon the spiritual and
practical skills of the TMPs
(traditional medicinal
practitioners), whose botanical
knowledge of plant species and
their ecology and scarcity are
invaluable. Throughout Africa,
the gathering of medicinal plants
was traditionally restricted to
TMPs or to their trainees (Photo
1). Knowledge of many species was
limited to this group through
spiritual calling, ritual,
religious controls and, in
southern Africa, the use of
alternative (hlonipha) names not
known to outsiders. |
Hedberg et al., (1982) observed
that the number of traditional
practitioners in Tanzania was estimated
to be 30 000 - 40 000 in comparison with
600 medical doctors (Table
1) (MP and TMP : total population
ratios were not given). Similarly, in
Malawi, there were an estimated 17 000
TMPs and only 35 medical doctors in
practice in the country (Anon., 1987).
Table
1. Ratios
of traditional medical
practitioners (TMPs) and medical
doctors to total population in
selected African countries.
| COUNTRY |
TMP
: TOTAL POP. |
MD
: TOTAL POP. |
REFERENCE |
NIGERIA
Benin City
National average |
1 : 110
? |
1 : 16 400
1 : 15 740 |
Oyenye & Orubuloye,
1983
Gestler, 1984 |
GHANAK
wahu district |
1 : 224 |
1 : 20 625 |
Anyinam, 1984 |
KENYA
Urban (Mathare)
Rural (Kilungu) |
1 : 833
1 : 146 - 345 |
1 : 987
1 : 70 000 |
Good, 1987
Family Health Institute,
1987 |
TANZANIA
Dar es Salaam |
1 : 350 - 450 |
? |
Swantz, 1984 |
ZIMBABWE
Urban areas
Rural areas |
1 : 234
1 : 956 |
?
? |
Gelfand et al, 1985 |
| SWAZILAND |
1 : 110 |
1 : 10 000 |
Green, 1985 |
SOUTH AFRICA
Venda area |
1 : 700 - 1 200 |
1 : 17 400 |
Savage, 1985
Arnold & Gulumian,
1987 |
*
so-called “homeland”
areas only
|
Economic and demographic
projections for most African countries
offer little grounds for optimism. A
shift from using traditional medicines to
consulting medical doctors, even if they
are available, only occurs with
socio-economic and cultural change,
access to formal education (Kaplan, 1976)
and religious influences (e.g. through
the African Zionist movements, which
forbid the use of traditional medicines
by their followers, substituting the use
of ash and holy water instead; Sundkier,
1961). Access to western biomedicine,
adequate education and employment
opportunities requires economic growth.
Unfortunately, most African countries are
affected by unprecedented economic
deterioration. Per capita income has
reportedly fallen by 4% since 1986,
whilst Africa’s foreign debt is
three times greater than its export
earnings. In Zambia, government spending
on education has fallen by 62% in the
last decade, and that on essential
pharmaceutical drugs by 75% from 1985 to
1989 (Zimbabwe Science News, 1989). At
the same time, the African population has
grown by 3% per annum, increasing the
difficulty of adequate provision of
Western-type health services. For this
reason, there is a need to involve TMPs
in national healthcare systems through
training and evaluation of effective
remedies, as they are a large and
influential group in primary healthcare
(Akerele, 1987; Anyinam, 1987; Good,
1987). Sustainable use of the major
resource base of TMPs - medicinal plants
- is therefore essential.
Customary controls
on medicinal plant gathering
The sustainable use of medicinal
plants was facilitated in the past by
several inadvertent or indirect controls
and some intentional management
practices.
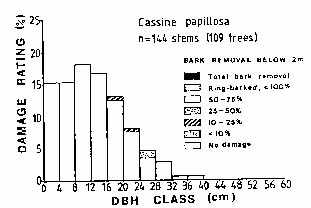 |
| Figure
1.
Assessment of debarking
damage to Cassine
papillosa (Celastraceae)
trees in an area where
subsistence harvesting
rather than commercial
exploitation is taking
place (Cunningham, 1988a)
|
|
Taboos,
seasonal and social restrictions
on gathering medicinal plants,
and the nature of plant gathering
equipment all served to limit
medicinal plant harvesting. In
southern Africa (and probably
elsewhere) before metal machetes
and axes were widely available,
plants were collected with a
pointed wooden digging stick or
small axe, which tended to limit
the quantity of bark or roots
gathered. For example,
traditional subsistence
harvesting of Cassine papillosa
bark causes relatively little
damage to the tree (Figure 1). |
Pressure on medicinal plant
resources has remained low in remote
areas and in countries such as Mozambique
and Zambia where the commercial trade in
traditional medicines has only developed
to a limited extent due to the small size
of major urban centres. Examples of
factors which have limited pressure on
species which would otherwise be
vulnerable to over-exploitation include:
(1) Taboos against the collection
of medicinal plants by menstruating
women in South Africa and Swaziland;
it is believed that this would reduce
the healing power of the plants
(Scudder and Conelly, 1985).
(2) The tendency in southern
Africa for women to practise as
diviners, while men practise as
herbalists (Berglund, 1976; Staugard,
1985). This limits the number of
resource users.
(3) The perceived toxicity of some
medicinal species which reduced their
use in the past: the level of
toxicity is sometimes given mythical
proportions. Synadenium cupulare for
example, is considered so toxic that
birds flying over the tree are
killed; special ritual preparations
are made in west Africa before the
bark of Okoubaka aubrevillei is
removed (Good, 1987).
(4) The traditional use of a
wooden batten for removal of bark
from Okoubaka aubrevillei - under no
circumstances may a machete or other
metal implement be used (Good, 1987).
For any society to institute
intentional resource management controls,
certain conditions have to be fulfilled:
(1) the resource must be of value
to the society;
(2) the resource must be perceived
to be in short supply and vulnerable
to over-exploitation by people;
(3) the socio-political nature of
the society must include the
necessary structures for resource
management.
Intentional resource management
controls have endured in Africa in
various forms and for various reasons and
some have affected the abundance and
availability of medicinal species. The
widespread practice in Africa of
conserving edible wild fruit-bearing
trees for their fruits or shade also
ensures availability of some traditional
medicines as several are multiple-use
species. For example the following six
trees are conserved for their fruit:
Irvingia gabonensis and Ricinodendron
heudelotii in west Africa (barks are used
for diarrhoea and dysentery); from
southern Africa Trichilia emetica
(enemas), Parinari curatellifolia
(constipation and dropsy), Azanza
garkeana (chest pains), and Sclerocarya
birrea (diarrhoea). Albizia
adianthifolia, used for enemas, is
conserved for its shade.
Protection of vegetation at grave
sites, for religious and spiritual
reasons, is a common feature in many
parts of Africa (including Kenya, Malawi,
South Africa and Swaziland) and an
important means through which biotic
diversity is maintained outside core
conservation areas. In south-eastern
Africa during the nineteenth century,
specific Zulu regiments were called up
annually to burn fire-breaks around the
grave sites of Zulu kings: these woodland
or forested sites were considered to be a
sanctuary for game animals (Webb and
Wright, 1986). An important feature of
vegetation conservation around grave
sites is that this practice is maintained
even under high population densities and
tremendous demand for arable land, for
example in Malawi. The practice might
possibly be strengthened through the
burial of prominent leaders in
conservation areas.
Religious beliefs have also helped to
ensure careful harvesting of Helichrysum
kraussii, an aromatic herb known as
impepho in Zulu which is widely burnt as
an incense in Natal. Diviners are careful
not to rip the plant out by its roots
(Cooper, 1979).
In Swaziland and South Africa, taboos
also restrict the seasonal (summer)
collection of Alepidea amatymbica roots,
Siphonochilus aethiopicus and Agapanthus
umbellatus rhizomes. In each case,
collection is restricted to the winter
months after seed set as summer gathering
is believed to cause storms and
lightning. In Zimbabwe, clearance has to
be obtained from ancestral spirits before
entering certain forests where Warburgia
salutaris occurs. In each of the above
cases (excepting Agapanthus umbellatus),
the species concerned are popular, scarce
and effective. These intentional
conservation practices may be due to the
century-old history of trade in these
plants in the southern African region.
Government legislation has played a
largely ineffective role in controlling
the use of medicinal plants in Africa.
Under colonial administration, religious
therapy systems practised by diviners
were equated with witchcraft and
legislated against almost everywhere
(Cunningham, 1990; Gerstner, 1938;
Staugard, 1985). In South Africa (and
possibly other parts of Africa) during
the colonial era, there were also
attempts to prohibit the sale of
traditional medicines within urban areas,
such as the efforts made by the Natal
Pharmaceutical Society in the 1930s in
Durban, South Africa. Apart from having
the temporary effect of driving informal
sector plant sellers and TMPs
underground, this kind of legislation has
been ineffective in reducing traditional
medicine use. Attempts to suppress
traditional medicine are not, however,
solely restricted to the colonial era: in
post-independence Mozambique, for
example, diviners involved in symbolic or
magico-medicinal aspects of traditional
medicine were sent to re-education camps
in an effort to do away with
“obscurantism” (Adjanohoun et
al., 1984).
Although forest legislation in most
African countries generally recognizes
the importance of customary usage rights
(including gathering of dead-wood for
fuel, felling poles and gathering latex,
gums, bark resins, honey and medicinal
plants) conservation land or certain
plant species are often set aside for
strict protection (Schmithusen, 1986). In
South Africa, for example, forestry
legislation was promulgated in 1914 for
the protection of economically important
timber species such as Ocotea bullata.
Specially protected status has been given
since 1974 to all species within the
families Liliaceae, Amaryllidaceae and
Orchidaceae due to their prominence in
the herbal medicine trade.
At best, this legislation has merely
slowed down the rate of harvesting.
Extensive exploitation within forest
reserves still occurs in South Africa.
One of the main reasons for this is that
legislation for core conservation areas
(CCAs) in the past has concentrated on a
“holding action” to maintain
the status quo and neglected to provide
local communities with viable
alternatives to collecting customary
plants.
Dynamics of the
commercial trade
If effective action is to be taken to
deal with the over-exploitation of
medicinal plants, there has to be a clear
understanding of the scale and complexity
of the problem.
Domestic
trade
Africa has the highest rate of
urbanization in the world, with urban
populations doubling every 14 years as
cities grow at 5.1% each year (Huntley et
al., 1989). In rural areas throughout
Africa, wild plant resources fulfill a
wide range of basic needs and are a
resource base harvested for informal
trade or barter, whereas in urban areas,
a much smaller range of species and uses
is found. In rural areas of the
Mozambique coastal plain for example, 76
edible wild plant species are used
(Cunningham, 1988a) but only five species
are sold in urban markets in Maputo.
Urbanization results in this general
reduction in the number of species and
the quantities of certain wild plant
resources used as people enter the cash
economy, and alternative foods, utensils
and building materials become available.
However, informal sector trade in two
categories of wild plant resources
continues to be very important in many
cities: fuelwood (alternative energy
sources such as electricity, gas and
paraffin are not available or affordable;
Eberhard, 1986; Farnsworth, 1988) and
medicinal plants.The range of
commercially sold medicinal species in
southern Africa remains wide despite
urbanization (over 400 indigenous species
in Natal, South Africa, for example;
Cunningham, 1990). Little attention has
been paid to the cultural, medical,
economic or ecological significance of
the herbal medicine trade, yet
traditional medicine sellers are a
feature of every African city (ECP/GR,
1983). Cities are concentrated centres of
demand drawing in traditional medicines
from outlying rural areas and across
national boundaries. Despite the
differences in volume and range of
species used, parallels can be drawn
between the trade in medicinal plants and
that in fuelwood:
(1) high proportions of people use
medicinal plants (70-80%) and
fuelwood (60-95%) (Leach and Mearns,
1988);
(2) high urban demand can
undermine the rural resource base by
causing the depletion of favoured but
slow growing species such as
Combretum (fuelwood, Botswana;
Kgathi, 1984) and Warburgia salutaris
(bark medicines, Zimbabwe);
(3) harvesting is a strenuous and
labour intensive activity with
financial returns, carried out by
rural people with a low level of
formal education and poor chance of
formal employment;
(4) supplies may be drawn from a
long distance away - from 200-500 km
for fuelwood in many African cities
(Leach and Mearns, 1989) and as far
as 800-1200 km for certain medicinal
plants in west Africa such as Entada
africana and Swartzia
madagascariensis or Synaptolepis
kirkii in southern Africa
(Cunningham, 1988a).
The herbal medicine trade is
characterized by two features. First,
from being almost solely an activity of
traditional specialists, medicinal plant
collection has now shifted to involve
commercial harvesters in the informal
sector, and (in South Africa at least)
formal sector traders (Table
2) who supply the large urban demand.
Women, rather than men, are increasingly
involved as non-specialist sellers of
traditional medicines, and this general
pattern is seen throughout Africa. In
rural areas and small villages, male and
female TMPs practise from their homes. In
larger villages, herbalists (mainly men)
dispense from a small quantity of
traditional medicines that they have
gathered themselves. In towns, larger
quantities of material are sold, some of
which are bought from commercial
harvesters, and in cities or large towns,
large quantities of plant material are
supplied by commercial harvesters and
sold through increasing numbers of
informal sector sellers (mainly women) to
urban herb traders or herbalists for
self-medication. Men drop out of
non-specialist sales as it becomes an
increasingly marginal activity, and only
persist as sellers of animal material.
Second, demand for traditional medicines
is highly species specific and
alternatives are not easily provided due
to the characteristics of the plant or
animal material, their symbolism, or the
form in which they are taken. These large
urban areas dictate prices, which are
kept low because of rising unemployment,
over-supply and cheap labour. Thus
nothing is paid towards the replacement
of the wild stocks.
In the stressful environment which is
a feature of many urban areas in Africa,
it is not surprising that demand has
increased for traditional medicinal plant
and animal materials which are believed
to have symbolic or psychosomatic value.
Table
2.
Number of traditional
medicine sellers (this excludes
chewing stick sellers) and herb
trader shops in selected African
urban areas, small towns (#),
large towns (*) and cities
(capital letters) from counts
during 1989 and early 1990.
| COUNTRY |
CITY/TOWN |
MARKET-BASED
SELLERS |
HERB
TRADERS |
| SOUTH AFRICA |
COTE D'IVOIRE
|
| ZIMBABWE |
| MOZAMBIQUE |
ZAMBIA
|
MALAWI
|
| SWAZILAND |
|
| DURBAN |
(3) |
ABIDJAN
Bouake* |
(4)
(1) |
| Harare* |
(2) |
| Maputo* |
(1) |
Lusaka*
Mongu# |
(2)
(1) |
Liiongwe*
Blantyre*
Zomba#
Mzuzu# |
(1)
(1)
(1)
(1) |
Mbabane*
Manzini# |
(1)
(1) |
|
| 392 |
22 |
270 |
111
64 |
4
26 |
107
37 |
| 36 |
25 |
11 |
| 25 |
19 |
6 |
16
3 |
5
3 |
11
0 |
3
8
3
2 |
3
8
3
2 |
0
0
0
0 |
3
4 |
2
2 |
1
2 |
|
| c.100 |
0
0 |
| 0 |
| 0 |
0
0 |
0
0
0
0 |
0
0 |
|
|
Traditional plant or animal
materials which bring luck in finding
employment, which guard against jealousy
(such as that engendered when one person
has a job whilst their peer group are
unemployed), or love-charms and
aphrodisiacs to keep a wife or girlfriend
are popular. Thus, employment options for
TMPs have increased with the stresses of
urban life. In addition, western-type
medical facilities have not been able to
cope with the rapidly growing urban
population. In Lagos, Nigeria, for
example, the ratio of medical doctors to
total population was 1 : 5000 in 1975
compared with 1 : 2000 in 1955 (Udo,
1982).
Traditional medical practitioners are
therefore attracted to urban centres
where employment benefits can be good, as
shown in studies in Nairobi (Kenya), Dar
es Salaam (Tanzania), Kampala (Uganda),
Kinshasha (Zaire) and Lusaka (Zambia)
(Good and Kimani, 1980) (Table 1).
In Zimbabwe there is a higher ratio of
TMPs to total population in urban areas
(1 : 234) than in rural areas (1 : 956)
(Gelfand et al., 1985). This is not
always the situation, however: in the
Kilungu district of Kenya, the ratio of
rural TMPs to people averaged 1 : 224,
while in urban Mathare, the overall ratio
was 1 : 883 (Good, 1987).
Box
1: Case study: The trade in chewing
sticks
Dentists are scarce in many parts of
Africa, particularly in rural areas. The
ratio of dentists: total population in
Ghana was 1 : 150 000 (compared to 1 : 3
000 in Great Britain) (Adu-Tutu et al.,
1979). Although diet plays a major role
in causing dental caries, the practice of
dental hygiene is also important. While
toothpaste and toothbrushes are widely
used by the sector of the population with
a high level of formal education,
toothpaste consumption is still low (e.g.
Adu-Tutu et al., 1979 in Ghana) and
chewing sticks are still in common use in
many parts of Africa, particularly west
Africa. Even when people would prefer to
use toothbrushes, they do not have access
to toothpaste due to high cost or
remoteness. Continued access to popular
and effective sources of chewing sticks,
which have anti-bacterial properties, is
important as a primary health care
measure.
While many hundreds of medicinal plant
species are used within a region, a
smaller number of the most popular
species accounts for much of the
commercial trade to urban areas. This
applies equally to chewing sticks. In
Mozambique for example, Euclea divinorum
and Euclea natalensis (Ebenaceae) are the
most commonly sold species, although
other species are used countrywide. In
Côte d’Ivoire, the most popular
sources of chewing sticks are Garcinia
afzelii and Garcinia kola and less
commonly used chewing sticks were
Zanthoxylum macrophyllum, Maytenus
senegalensis, Pycnanthus angolensis and
Enantia polycarpa. In Cameroon, only
Garcinia mannii and Randia acuminata were
the basis of a chewing stick
“cottage industry” (Staugard,
1985). Similarly, in southern Ghana, from
a sample of 880 people interviewed, six
species (distinguished by four local
names) accounted for 86% of all usage and
the majority of the commercial sales. The
majority of all these respondents depend
on bought material rather than collecting
it themselves, irrespective of size of
settlement they live in or their
educational status (Figure 2). The
species used were: nsokodua (Garcinia
afzelii and G. epunctata (51.1%; 597
people); tweapea (Garcinia kola (18.7%;
218); sawe (Acacia kamerunensis and
Acacia pentagona (9.2%; 108); and
owebiribiri (Teclea verdoorninana (6.7%;
77).
Figure 2.
A.
Acquisition of usual chewing
stick by buying (shaded columns)
and collecting (open columns)
among people of various sizes of
settlement (after Cunningham,
1988a)
B. Acquisition of usual chewing
stick by buying (shaded columns)
and collecting (open columns)
among people differing in
educational background (after
Adu-Tutuet al. , 1979).
|
International
trade
The herbal medicine trade is booming
business worldwide. In India, for
example, there are 46 000 licensed
pharmacies manufacturing traditional
remedies, 80% of which come from plants
(Alok, 1991). Another example is Hong
Kong, which is claimed to be the largest
market in the world, importing over US$
190 million annually (Kong, 1982). In
Durban (South Africa), in 1929 there were
only two herbal traders; by 1987, there
were over 70 herbal trader shops
registered. The species specific nature
of the demand for medicinal plants is
responsible for generating long distance
trade across international boundaries.
According to Malla (1982), 60-70% of the
medicinal herbs collected in Nepal are
exported to India, with 85-200 tons
exported annually between 1972 and 1980.
Similarly the Hong Kong market imports
Aquilaria heart-wood for incense
manufacture from rain forest in Thailand
and Malaysia. This is devastating
Aquilaria populations in core
conservation areas such as Khao Yai
National Park, Thailand (Cunningham,
pers. obs.; Cunningham, 1988a;
Cunningham, 1988b). Africa is no
exception to this pattern and an informal
sector trade in medicinal plants spans
long distances:
(1) the roots of Swartzia
madagascariensis and Entada africana
are traded 500-800 kms from Burkina
Faso and Mali to Abidjan, Côte
d’Ivoire;
(2) the roots of Synaptolepis
kirkii are traded 1200 km from the
southern border of Mozambique and
South Africa, via Johannesburg, to
Maseru (Lesotho);
(3) the bark of Warburgia
salutaris is traded from Swaziland to
Johannesburg (South Africa) and
Namaacha (on the Swaziland/Mozambique
border) to Maputo (Mozambique);
(4) the roots of Alepidea
amatymbica and bark of Warburgia
salutaris are traded from the Eastern
Highlands (Zimbabwe) to urban centres
in the west of the country such as
Bulawayo;
(5) mail-order trade in
traditional medicines is common in
South Africa (Figure 3).
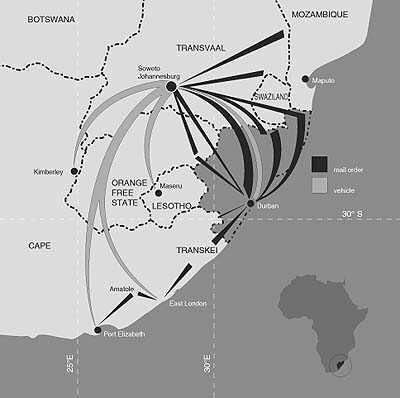 |
| Figure
3. Long
distance trade in Natal province,
South Africa, from the remotest
rural areas to major urban
centres through formal and
informal trade networks,
including mail order sales. |
An average of 25% of
prescription drugs sold in the USA during
the period 1959-1973 contained active
principles extracted from higher plants
(Farnsworth and Soejarto, 1985). Many of
these are derived from the same source as
those used in traditional medicine. On a
global scale, 74% of these chemicals have
similar or related uses in traditional
medicine (Farnsworth, 1988). Similarly,
many African plant species are the source
of a number of active ingredients for the
export market (Table 3, Photo 2). Because
of the low price demanded by plant
traders, even when technology for
chemical synthesis is available, it can
be cheaper for pharmaceutical companies
to continue to extract the active
ingredients from plants. In the
mid-1970s, for example, synthesis of
reserpine cost $1.25 g-1, compared to a
cost of $0.75 per g-1 for commercial
extraction from Rauvolfia vomitoria roots
(Oldfield, 1984).
Table
3. Indigenous
plants that are harvested as a
source of active ingredients for
export purposes, indicating what
part of the plant is harvested
for extraction of active
ingredients and whether the
plants are used in traditional
medicine or not.
| |
|
|
|
|
| SPECIES |
PART
USED |
INGREDIENT |
SOURCE
AREA |
TM |
| Adhatoda robusta |
? |
? |
Ghana (1) |
- |
| Allanblackia
floribunda |
fruit |
fat** |
Cote d'ivoire
(2) |
* |
| Ancistrocladus
abbreviatus |
? |
? |
Ghana (1) |
- |
| Corynanthe
pachyceras |
? |
corynanthine |
Ghana (1) |
* |
| |
|
corynathidine |
|
|
| |
|
yohimbine |
|
|
| Dennetia
tripetala |
? |
? |
Ghana (1) |
- |
| Duparquetia
orchidacea |
? |
? |
Ghana (1) |
* |
| Griffonia
simplicifolia |
seed |
BS11 lectin |
Côte d’
Ivoire,
Cameroon &
Ghana (1,2,5) |
* |
| Harpagophytum
procumbens |
root |
glucoiridoids |
Namibia (3) |
* |
| Harpagophytem
zeyheri |
root |
glucoiridoids |
Namibia (3) |
* |
| Hunteria eburnea |
bark |
eburine and
other alkaloids |
Ghana (1) |
* |
| Jateoriza
palmata |
root |
palmatrin |
Tanzania (4) |
* |
| |
|
jateorhizine
colambamine |
|
|
| Pausinystalia
johimbe |
bark |
yohimbine |
Cameroon (5) |
* |
| Pentadesma
butryacea |
fruit |
fat** |
Côte
d’Ivoire (2) |
* |
| Physostigma
venenosum |
fruit |
physostigmine |
Côte
d’Ivoire (2) |
* |
| |
|
(eserine) |
Ghana (1) |
* |
| Prunus africana |
bark |
sterols |
Cameroon, Kenya |
* |
| |
|
triterpenes
n - docosanol |
Madagascar (6)
|
* |
| Rauvolfia
vomitoria |
root |
reserpine |
Zaire, Rwanda, |
* |
| |
|
yohimbine etc. |
Mozambique |
|
| Strophanthus
spp. |
fruit |
ouabain |
West Africa |
* |
| Voacanga
africana |
seed |
voacamine |
Côte
d’Ivoire, |
* |
| |
|
|
Cameroon,
Ghana (1,2,5) |
|
| Voacanga
thouarsii |
seed |
voacamine |
Cameroon(1,2,5) |
* |
Note
:
Fat from Allanblackia stuhlmannii
fruits,used in soap making and
cosmetics industry (Lovett,
1988). Use of products from
Jateorhiza now limited mainly to
veterinary medicine (Oatley,
1979).References: 1 = (Abbiw,
1990); 2 = L. Ake Assi, pers.
comm.; 3 = (Nott, 1986); 4 = J.
Seyani, pers. comm.; 5 = (FAO,
1986); 6=(Catalano et al., 1985)
|
According to the
UNCTADD/GATT International Trade Centre,
the total value of imports of medicinal
plants for OECD countries, Japan and the
USA increased from US$ 335 million in
1976 to US$ 551 million in 1980 (Husain,
1991). Of the 200 tons of Harpagophytum
procumbens and H. zeyheri tubers exported
annually from Namibia, Germany imported
80.4%, with the remaining 12.8% sold to
France, 1.9% to Italy, 1.5% to USA, 1% to
Belgium and 1.2% sold locally or to South
Africa (Nott, 1986). Unfortunately, the
low prices paid for the plants do not
cover replacement or resource management
costs, and as such, major importers
demanding high volumes of plant material
are contributing to the decline of
medicinal plant species in Africa.
 |
| Photo
2.
Medicinal plant seller at a
market in Abidjan, Côte
d’Ivoire, showing the
dominance of fresh leaf material
as a source of herbal medicines. |
|